Reactions of aldehydes and ketones
Reactions of aldehydes and ketones: Overview
This topic covers concepts, such as, Chemical Properties of Aldehydes and Ketones,Nucleophilic Addition Reactions of Aldehyde and Ketones,Mechanism of Nucleophilic Addition Reactions of Aldehyde and Ketones etc.
Important Questions on Reactions of aldehydes and ketones
Compound which gives cannizzaro reaction?
Given reaction is an example of
Given reaction is an example of
Oxidation number of carbon changes from
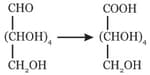
Reagent responsible for given conversion will be
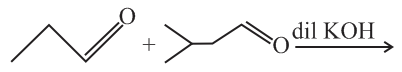
Total number of aldol condensation products are (excluding stereoisomer)
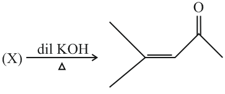
Structure of is:
Aldol addition can be
Aldol addition can be
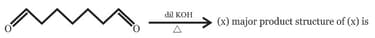
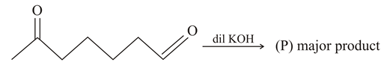
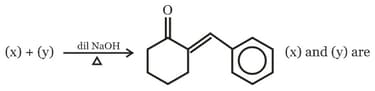
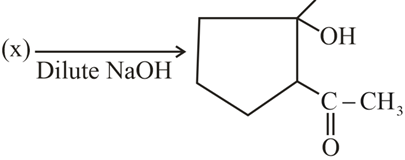 ; (X) is
; (X) is
Reduction of the carbonyl group of aldehydes and ketones on treatment with hydrazine followed by heating with potassium hydroxide is known as
Name the following reactions:
The carbonyl group of aldehyde and ketones is reduced to group on treatment with zinc amalgam and concentrated hydrochloric acid.
Aldehydes that do not contain any hydrogen atom undergo self-oxidation and reduction reaction on treatment with concentrated alkali.
Write the name of following chemical reactions.
(i) Aldehydes which do not contain an hydrogen atom, when treated with a concentrated alkali solution undergo self-oxidation–reduction.
(ii) Carboxylic acids react with chlorine or bromine in the presence of phosphorous to give compounds in which a hydrogen atom is replaced by a halogen atom.
A redox reaction in which two molecules of an aldehyde react to produce a primary alcohol and a carboxylic acid using a hydroxide base is called:
Aldehydes, which do not contain an -hydrogen atom, when treated with concentrated alkali solution undergoes self –oxidation and reduction. The reaction is known as:
Identify to in the following series of reactions:
In Cannizzaro reaction given below
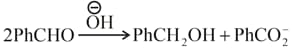
Then, which of the following is correct about the rate determining step?